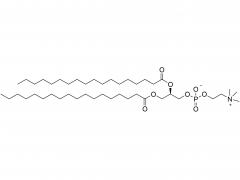
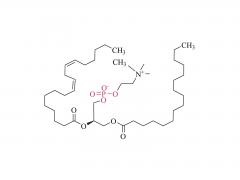
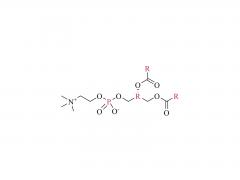
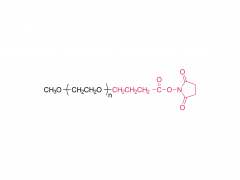
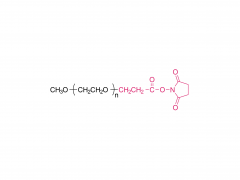
Im Bereich der Biomedizin, insbesondere bei den in den letzten Jahren stark angestiegenen mRNA-Impfstoffen und Nukleinsäure-Medikamenten, spielt DMG-PEG 2000 eine unverzichtbare Rolle. Als eine der Schlüsselkomponenten des Lipid-Nanopartikel-Systems (LNP)
DMG-PEG 2000 spielt
Es spielt eine entscheidende Rolle bei der Verkapselung, dem Schutz und der gezielten Verabreichung von Nukleinsäure-Arzneimitteln. Tatsächlich ist es eine der Schlüsselkomponenten des LNP-Systems im COVID-19-mRNA-Impfstoff (BNT162b2) von Pfizer/BioNTech, was dieser ursprünglich spezialisierten Verbindung auch große Aufmerksamkeit beschert hat.
Obwohl DMG-PEG 2000 in der Formulierung von LNP üblicherweise nur einen sehr geringen molaren Anteil (ca. 1,5–2 %) ausmacht, ist seine Rolle entscheidend und vielschichtig. Erstens gewährleistet es die räumliche Stabilität und verhindert die Aggregation: Die PEG-Kette von DMG-PEG 2000 bildet eine hydrophile „Schutzschicht“ auf der Oberfläche der LNP. Durch sterische Hinderung wird die Aggregation und das Verschmelzen der Partikel während der Lagerung und der Verabreichung in vivo wirksam verhindert, wodurch die Einheitlichkeit und Stabilität der LNP-Partikelgröße erhalten bleibt. Diese Funktion ist entscheidend für die Gewährleistung der Konsistenz und Stabilität zwischen verschiedenen Arzneimittelchargen.
Zweitens verlängern die PEG-Schutzschicht die Blutkreislaufzeit und verleihen den LNPs eine gewisse „Unsichtbarkeit“: Sie reduziert die Adsorption von Blutbestandteilen wie Serumproteinen an der Oberfläche der LNPs (und verringert so die Bildung von Proteinkoronen). Dadurch entgehen die LNPs der Erkennung und dem Abbau durch das mononukleäre Phagozytensystem (MPS, z. B. Lebermakrophagen). Dies verlängert die Verweildauer der LNPs im Körper signifikant und erhöht ihre Chancen, den Wirkort zu erreichen. Diese Eigenschaft verleiht den LNPs die „Unsichtbarkeit“. Im Vergleich zu anderen PEG-modifizierten Lipiden sind PEG-Lipide mit kurzen Acylketten wie DMG-PEG2000 so konzipiert, dass sie in vivo schnell dissoziieren und so die Freisetzung der verkapselten Wirkstoffe am Zielort ermöglichen.
Neben seiner Anwendung in mRNA-Impfstoffen findet DMG-PEG 2000 auch breite Verwendung in den Verabreichungssystemen anderer Nukleinsäure-Arzneimittel. Es wird für die siRNA-Arzneimittelverabreichung und die Gentherapie eingesetzt. Darüber hinaus kann es zur Verkapselung und Verabreichung von Nukleinsäure-Arzneimitteln wie Plasmid-DNA (pDNA), microRNA (miRNA) und CRISPR-Cas9-Geneditierungssystemen verwendet werden. Bei der Herstellung von mit siRNA transfizierten Liposomen kann DMG-PEG 2000 die Transfektionseffizienz verbessern. Es kann auch bei der Herstellung von Lipidnanopartikeln für die orale Verabreichung von Plasmid-DNA in vivo eingesetzt werden, um die Schleimhautpermeabilität und die Verabreichungseffizienz der Nanopartikel zu erhöhen.
Obwohl der Anteil von DMG-PEG 2000 in der LNP-Formulierung gering ist, hat er einen erheblichen Einfluss auf die Eigenschaften und Funktionen der LNP. Durch die Anpassung des DMG-PEG-2000-Gehalts in der Formulierung lassen sich Partikelgröße, Oberflächenladung, Stabilität und In-vivo-Verteilung der LNP präzise steuern. Beispielsweise verringert eine Erhöhung des DMG-PEG-2000-Anteils in der Regel die Partikelgröße der LNP, jedoch können zu hohe Mengen die Stabilität und Transfektionseffizienz beeinträchtigen. Diese Steuerungsmöglichkeit erlaubt es Forschern, LNP-Formulierungen für verschiedene Anwendungsszenarien zu optimieren.
Im Vergleich zu dem häufig verwendeten PEG-modifizierten Lipid DSPE-MPEG2000 bietet DMG-PEG 2000 einzigartige Anwendungsvorteile. DSPE-MPEG2000 wird häufig zur Herstellung langzirkulierender Liposomen, wie beispielsweise des Doxorubicin-Liposoms DOXIL, eingesetzt. DMG-PEG 2000 hingegen erzielt bessere Ergebnisse hinsichtlich Leber-Targeting und Gen-Silencing-Effizienz und ist daher das bevorzugte Material für langzirkulierende kationische Liposomen von RNA-Arzneimitteln. Dieser Unterschied beruht hauptsächlich auf den unterschiedlichen chemischen Strukturen der beiden Lipide: DMG-PEG 2000 besitzt eine kürzere C14-Acylkette, während DSPE-MPEG2000 eine längere C18-Acylkette aufweist. Dies beeinflusst die Verweildauer in der Lipiddoppelschicht und die Interaktion mit der biologischen Membran.
Im Bereich der Arzneimittelforschung und -entwicklung bietet die Anwendung von DMG-PEG 2000 eine wichtige technische Unterstützung für die Entwicklung von Nukleinsäure-Arzneimitteln. Aufgrund ihres hohen Molekulargewichts, ihrer negativen Ladung und ihrer leichten Abbaubarkeit durch Nukleasen stellen Nukleinsäure-Arzneimittel erhebliche Herausforderungen beim direkten Transport zum Wirkort in den Zellen dar. LNP-Systeme, insbesondere solche mit DMG-PEG 2000, haben diese Transportprobleme effektiv gelöst. Es schützt die Nukleinsäuremoleküle nicht nur vor dem Abbau, sondern fördert auch die zelluläre Aufnahme und den Austritt aus Endosomen, wodurch Nukleinsäure-Arzneimittel ihre therapeutische Wirkung entfalten können.
Mit der Entwicklung personalisierter Medizin und Gentherapie eröffnen sich für DMG-PEG 2000 vielfältige Anwendungsmöglichkeiten in der Präzisionsmedizin. Durch Modifizierung des PEG-Terminus mit zielgerichteten Liganden (wie Antikörpern, Peptiden oder niedermolekularen Verbindungen) kann LNP die Fähigkeit erhalten, spezifische Zellen oder Gewebe gezielt anzusteuern. Obwohl DMG-PEG 2000 selbst eine inerte Methoxygruppe besitzt, lässt sich die Zielfunktion durch chemische Modifizierung oder die Verwendung anderer funktionalisierter Derivate (wie DMG-PEG-COOH) erzielen. Diese gezielte Wirkstofffreisetzung kann die Wirkstoffkonzentration am Wirkort erhöhen und Nebenwirkungen auf gesundes Gewebe reduzieren. Dies stellt eine wichtige Richtung für die zukünftige Entwicklung von Wirkstoffträgersystemen dar.
Insgesamt hat DMG-PEG 2000 als Schlüsselkomponente von Nukleinsäure-Arzneimittelverabreichungssystemen seinen Wert in der modernen Medizin unter Beweis gestellt. Mit der kontinuierlichen Entwicklung neuer Nukleinsäure-Arzneimittel und der fortlaufenden Optimierung der LNP-Technologie wird erwartet, dass DMG-PEG 2000 und seine Derivate in weiteren Therapiebereichen eine wichtige Rolle spielen und neue Lösungen für die Behandlung vieler schwer behandelbarer Erkrankungen bieten werden.